The synthetic carbon-neutral pathways share a common element: the glycolate reduction module, which is composed of two novel reactions to reduce glycolate to glycolaldehyde. In the paper, published in the Proceedings of the National Academy of Sciences, we describe how they experimentally realized a carbon-neutral shunt by engineering the enzymatic activities needed for these two new-to-nature reactions.
By means of computational design and directed evolution we engineered:
- A glycolyl-CoA synthetase starting from on an acetyl-CoA synthetase. Based on the speculation that the coliacetyl-CoA synthetase (EcACS) may also accept glycolate, we tested and confirmed its ability to ligate glycolate to CoA with a low catalytic efficiency. We subsequently applied computational design to engineer a higher glycolyl-CoA and lower ACS efficiency. The resulting enzyme (ACS19), compared with the starting EcACS, has more than 60 point-mutations and resulted in approximately a 16-fold shift in favor of glycolate.
- A glycolyl-CoA reductase starting from a propionyl-CoA reductase. The Rhodopseudomonas palustrispropanediol utilization protein (RpPduP) shows promiscuous activity with acetyl-CoA and other CoA-ester substrates. The FutureAgriculture team used a combination of structure-based design and high-throughput screening to select the right variant that shows an enhanced selectivity for glycolyl-CoA and the ability to use NADPH over NAD+, thereby favoring reduction over oxidation. This variant, named GCR exhibits NADPH-preference as well as ∼12-fold improved selectivity for glycolyl-CoA over acetyl-CoA compared with wild-type RpPduP.
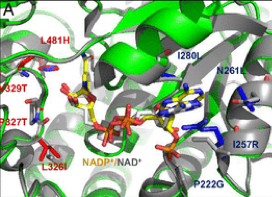
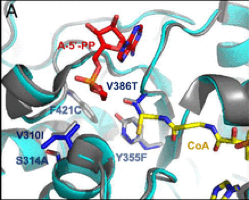
Figure1. Point mutations implemented to the selected enzymes in order to enhance their desired activity. From right to left: ACS19 superimposed to EcACS and GCR to RpPduP.
We further examined whether the combination of the two engineered enzymes could efficiently convert glycolate to glycolaldehyde. When ACS19 was combined with GCR, glycolate was efficiently reduced to glycolaldehyde without substential inhibition by NAD+. Engineered ACS19 and GCR, therefore, comprised a successful glycolate reduction module.
This module was then combined with three existing enzymes (EcFsaA-EcKdsD-RsPRK) to convert glycolate to RuBP, thus providing a proof of principle for the carbon-conserving A5P photorespiration bypass. We were able to produce RuBP from glycolate with an overall turnover rate of 0.05/s per enzyme for the entire pathway. The rate of glycolate reduction as measured by NADPH consumption matched the rate of RuBP production, indicating a tight coupling between the reduction and condensation modules, with no waste of reducing potential or off-target reactions in this in vitro setup. Such in vitro realization of a carbon-conserving pathway, as provided in this study, provides the foundation for its realization in photosynthetic organisms.






